Smart Polymers: Shape Memory and Self-Healing Materials
By Mufaddal Shakir
8/27/20258 min read
In a world where materials should be able to deliver something more than just to exist, smart polymers have appeared as groundbreaking players. For instance, think of a material that can change its shape with respect to temperature, release medicine when it locates a disease marker, or contract like muscle when stimulated by electricity. These are not any imaginary things, but they are real applications of smart polymers in today's world.
So, what truly are these amazing materials, and why are they gaining importance in industries from medicine to robotics? Let's dive in.
Recent research in the field of smart polymers:
1. pH & glucose dual-responsive phenylboronic-acid hydrogels for smarter insulin delivery
2. Electrothermally controlled origami via 4D printing (shape-shifting printed structures)
3. Stimuli-responsive smart polymers built around functional dyes (light/sensing/actuation)
4. Multi-material vat photopolymerization for 4D printing of stimuli-responsive polymers
5. “Smart” thermoresponsive copolymers for selective rare-earth (lanthanide) recovery
1) What are Smart Polymers?
Smart polymers, sometimes also referred to as stimuli-responsive polymers, are advanced materials that experience significant and reversible changes in their physical or chemical properties in response to external stimuli. These polymers respond to external environmental factors such as temperature, light, pH, electric or magnetic fields, biological molecules, ionic strength by changing their shape, permeability, solubility, or phase.
This smart behaviour allows them to perform different tasks such as self-healing, actuation, drug release, or colour change, depending upon the stimulus.
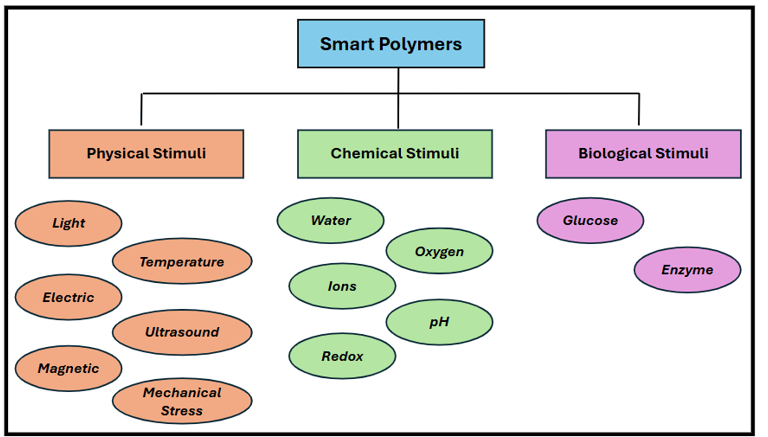
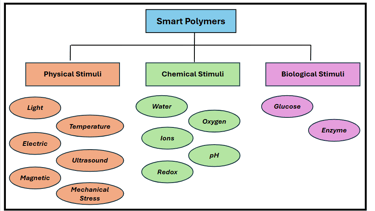
Classification of Smart Polymers Based on Stimuli.
2) Types of Smart Polymers Based on Stimuli
a) Temperature-Responsive Polymers
Temperature-responsive polymers, also known as thermoresponsive polymers are polymers that exhibit drastic changes in their physical properties with respect to temperature. These polymers show a miscibility gap in their phase diagram. An upper critical solution temperature (UCST) or lower critical solution temperature (LCST) exists depending on the miscibility gap which is either found at higher or lower temperatures. The research is mostly focussed on polymers that display thermoresponsivity in aqueous solution. Only a few commercial applications exists, for instance, cell culture plates coated with an LCST-polymer. Another example include Poly(N-isopropylacrylamide) (PNIPAM) which becomes insoluble in water above approximately 32 ℃, making it optimal for tissue engineering and drug delivery.
b) pH-Responsive Polymers
These smart polymers can change their properties, such as solubility or volume depending on the alkalinity and acidity of the environment. These polymers mostly contain carboxylic acid (-COOH) or amine groups (-NH2) that become protonated or deprotonated depending on the pH, leading to the observed changes. The alteration in the protonation state of these groups affects the polymer's overall charge and hydrophobicity/hydrophilicity, leading to shrinking, swelling or other structural changes. Common examples include Polyacrylic acid (PAA) which contains carboxylic acid groups and is utilized in applications like hydrogels and drug delivery. Another example includes chitosan, a naturally derived polymer with pH-responsive properties that makes it suitable for biomedical applications.
c) Light-Responsive Polymers
Light-responsive polymers are smart materials that can alter their physical or chemical properties when exposed to different wavelength of lights, including visible, UV, and near infrared. These polymers contain specific molecular units called chromophores, such as azobenzene, spiropyran, and stilbene that react to light exposure. Depending on the polymer structure and chromophore, the changes induced by light can be reversible or irreversible. Specific examples include azobenzene containing polymers that are known for their reversible photoisomerization between trans and cis forms when exposed to visible and UV light, which can be utilized for actuation. Another example includes light-responsive hydrogels, containing photosensitive moieties, that can be used for various applications including tissue engineering, drug release, and other medical applications.
d) Electric & Magnetic-Field Responsive Polymers
Electric and magnetic field responsive polymers are smart polymers that respond to electric or magnetic fields by changing their shape or generating heat. Electric field-responsive polymers or Electrorheological (ER) polymers, when exposed to an electric field, display changes in their properties, such as stiffness or viscosity. This effect occurs due to polarization of particles dispersed within a fluid, leading to chain formation and increased flow resistance. Some examples include dielectric gels and elastomers, ionic and conductive polymers. ER fluids can be utilized in brakes, clutches, and dampers, providing controlled resistance to motion. Magnetic field-responsive polymers or Magnetorheological (MR) polymers, display same behaviour as ER polymers but respond to magnetic fields. Some examples include MR fluids, ferrofluids, magnetic elastomers, and magnetic gels. The applications of MR fluids can be seen in shock absorbers, haptic feedback systems, vibration control devices, biomedical, and soft robotics.
e) Biologically-Responsive Polymers
Biologically-responsive polymers are smart materials that can change their properties such as structure or solubility in response to biological stimuli such as temperature, pH, or the presence of specific enzymes or molecules. The responsiveness of these polymers is attained by adding specific chemical groups or linkages into the structure of polymers that are sensitive to particular stimuli. Some examples include microgels, hydrogels, and nanoparticles which can be designed to respond to different biological signals.
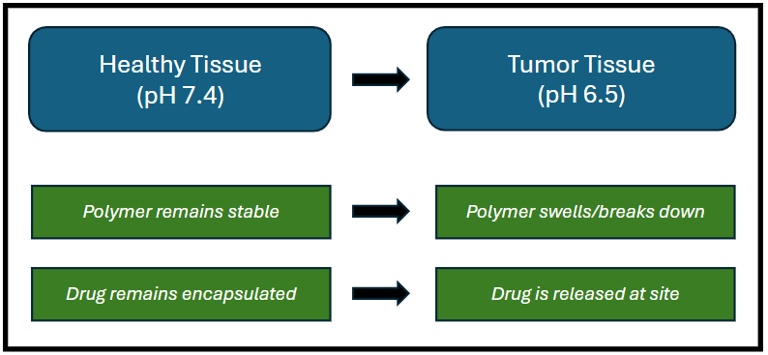
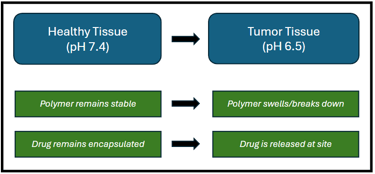
Mechanism of a pH responsive drug delivery smart polymer.
3) How Are Smart Polymers Made?
Smart polymers are manufactured through chemical synthesis processes that incorporate responsive functional groups or nano-structural elements into the backbone of a polymer. These functional elements provide the polymer its ability to respond to external stimuli (e.g., light, pH, temperature, etc.). The selection of manufacturing technique depends on the type of stimulus response required, polymer base material, intended application (biomedical, industrial, etc.), and processing scalability.
a) Traditional Polymerization Methods
These are traditional ways of making polymer chains, but with a twist: researchers use functional monomers or crosslinkers that respond to stimuli.
Free Radical Polymerization:
Polymer chains grow through free radicals initiated by heat, light, or chemicals. Simple, cheap, and compatible with a wide variety of monomers. Adding monomers like N-isopropylacrylamide (PNIPAM) for temperature responsiveness or acrylic acid for pH responsiveness. Example: pH-responsive hydrogels for oral drug delivery.
Controlled/Living Radical Polymerization:
Provides precise control over polymer molecular weight, architecture, and functionality. This method makes block copolymers with one responsive block and one stable block. Example: Block copolymer micelles that release drugs at low pH.
b) Grafting Methods
Grafting techniques are important for developing smart polymers by attaching specific polymer chains to a base polymer, modifying its properties for required functionalities. These techniques, such as "grafting to," "grafting from," and "grafting through," involve chemical, radiation, or enzymatic means to create covalent bonds between the grafted chains and the backbone polymer. By carefully selecting the grafting technique and the grafted polymer, researchers can alter the properties of smart polymers for a wide range of advanced applications.
c) Physical Incorporation of Responsive Elements
Instead of chemically bonding responsive groups, they are physically embedded. Some examples include incorporating magnetic nanoparticles into a polymer resin to produce magnetic-responsive composite or dispersing light-sensitive dyes like azobenzene in a polymer film.
d) Electrospinning
This technique uses high-voltage electric fields to produce fine polymer fibers from a solution. It produces nanofibers with a huge surface area, useful for sensors, drug delivery, and tissue engineering. By incorporating responsive nanoparticles or functional monomers during spinning, we can get the desired smart polymer.
e) Additive Manufacturing
3D or 4D printing of objects that change shape or function over time in response to a stimulus. Allows for custom implants, actuators, and responsive devices. Research is still in an early stage for utilizing 3D printing for optimizing printing parameters and processes for large-scale manufacturing of smart polymers.
4) Properties of Smart Polymers
a) Reversibility
Unlike traditional polymers, smart polymers return to their original state once the stimulus is removed.
This reversible nature is critical for repeated use (e.g., sensors, drug delivery systems).
b) Biocompatibility & Biodegradability
Many smart polymers (especially for medical use) are designed to be non-toxic, non-immunogenic, and biodegradable.
Ensures safe use in drug carriers, implants, and tissue scaffolds.
c) Self-Healing Ability
Some smart polymers can repair microscopic cracks or damage when triggered by heat, UV light, or chemical reaction.
Important for coatings, structural materials, and electronics.
d) Mechanical Adaptability
Ability to change stiffness, shape, or elasticity dynamically.
Example: Shape Memory Polymers (SMPs) return to a pre-set shape when heated.
e) Selectivity & Specificity
Respond only to particular stimuli, making them highly targeted.
Example: Glucose-sensitive hydrogels release insulin only in response to blood sugar levels.
f) Durability & Stability
Must maintain responsiveness over multiple cycles of stimulation.
Important for industrial and biomedical applications.
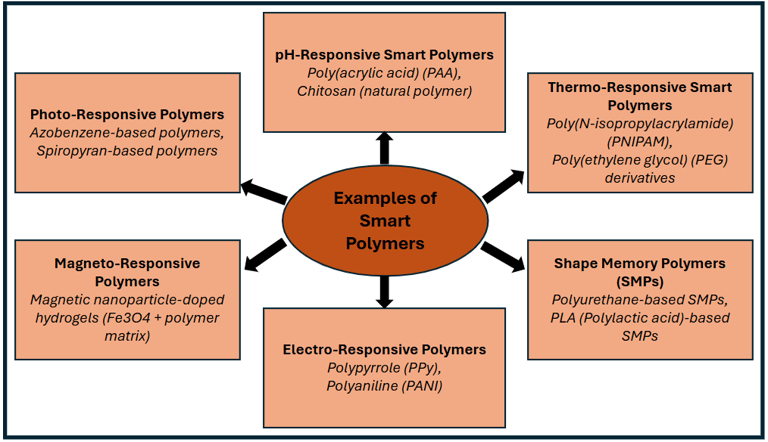
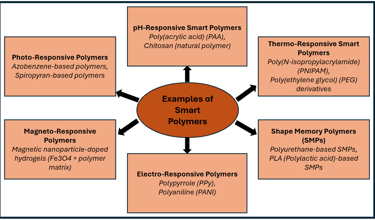
Examples of different smart polymers.
5) Applications of Smart Polymers
a) Biomedical & Healthcare
Drug Delivery Systems – pH and temperature responsive hydrogels release drugs only when they reach the target site. For example, glucose-responsive hydrogels for insulin release in diabetes management.
Tissue Engineering – Shape-memory polymers act as scaffolds that can change form during implantation and support tissue regeneration.
Self-Healing Implants – Polymers that repair microcracks in situ, extending the life of implants.
Biosensors – Optical or electrochemical sensors with smart polymer coatings for detecting biomarkers like glucose, lactate, or toxins.
b) Textiles & Wearable Technology
Thermochromic Clothing – Color-changing fabrics that react to sunlight or temperature of the body.
Moisture-Responsive Sportswear – Expands or contracts pores to regulate breathability and sweat evaporation.
Smart Medical Garments – Monitor heart rate, hydration, or blood oxygen using polymer-based sensing fibers.
c) Automotive & Aerospace
Self-Healing Car Paints – Shape-memory polymers that remove scratches under heat or sunlight.
Variable-Stiffness Components – Interior or structural parts that change rigidity in response to vibrations or temperature.
De-icing Systems – Electroresponsive coatings that prevent ice build-up on airplane wings.
d) Packaging Industry
Time–Temperature Indicators – Smart labels that change color if food is stored improperly.
Moisture-Responsive Films – Packaging that changes permeability to control humidity for fresh produce.
Antimicrobial Coatings – Polymers that release active agents only when triggered by moisture or bacteria.
e) Energy & Electronics
Flexible Displays – Electroactive polymers for bendable phone and tablet screens.
Artificial Muscles – Used in robotics and prosthetics for lifelike motion.
Self-Cleaning Solar Panels – Hydrophobic smart coatings that repel dust and dirt.
Energy Harvesting – Piezoelectric polymer films generating electricity from motion or vibration.
f) Environmental & Water Treatment
Smart Membranes – Open/close pores for selective filtration in wastewater treatment.
Oil–Water Separation – Hydrophobic/oleophilic coatings that switch properties under a stimulus.
Pollutant Sensors – Color-changing polymers detecting heavy metals or toxins.
6) Challenges in the Development and Utilization of Smart Polymers
a) High Processing and Synthesis Costs
Smart polymers require complex monomers, advanced polymerization techniques, or addition of costly nanoparticles or functional groups. Mass production is expensive, which limits the commercial use of smart polymers outside high-value applications like biomedical devices.
b) Long-Term Stability
The responsiveness of smart polymers generally relies on delicate molecular structures (e.g., photo-isomerizable units, reversible covalent bonds, hydrogen bonds). Exposure to moisture, oxygen, UV light, or biological fluids can deteriorate their properties over time.
c) Slow or Inefficient Response Time
The response time of smart polymers can be slow, especially in thick films or bulk samples. A delay in response time might not be acceptable for applications such as drug release or real-time biosensing.
d) Limited Biocompatibility
A few smart polymers may not be naturally safe for the body; some release toxic degradation products or trigger immune responses. This limits their utilization in drug carriers, implants, or tissue scaffolds unless modified or coated.
e) Reproducibility in Real-World Conditions
Laboratory trials often utilize ideal conditions (controlled pH, pure solvents), but real working environments have complex mixtures that can interfere with responsiveness which might decrease performance in actual use.
f) Integration with Existing Systems
Smart polymers often need to work with electronics, microfluidics, or mechanical devices. Compatibility issues can arise due to thermal, chemical, or mechanical mismatches.
Smart polymers are not just any materials, but they are solutions. From intimating biological systems to empowering futuristic technologies, these smart materials depict what's possible when chemistry meets creativity. Smart polymers represent one of the most exciting intersections of functionality and innovation. Whether you are an engineer, researcher, or curious reader, keep an eye on this space because materials of the future will think for themselves.
Further reading:
Yang, K., Bo, H., Ma, D., Peng, M., Liu, Q., Heng, Z., Gu, Z., Liu, X., & Chen, S. (2024). pH and glucose dual-responsive phenylboronic acid hydrogels for smart insulin delivery. Soft Matter, 20, 8855–8865. https://doi.org/10.1039/D4SM01004C
Wang, Y., Ye, H., He, J., Ge, Q., & Xiong, Y. (2024). Electrothermally controlled origami fabricated by 4D printing of continuous fiber-reinforced composites. Nature Communications, 15, Article 2322. https://doi.org/10.1038/s41467-024-46591-3
Alam, F., & El-Atab, N. (2024). Multi-material 4D printing employing stimuli-responsive polymer composites using vat photopolymerization. Virtual and Physical Prototyping, 20(1). https://doi.org/10.1080/17452759.2024.2444576
Bonetti, L., Cobianchi, A., Natali, D., Pandini, S., Messori, M., Toselli, M., & Scalet, G. (2024). Solvent-triggered shape change in gradient-based 4D printed bilayers: Case study on semi-crystalline polymer networks. Soft Matter, 20, 4544–4547. https://doi.org/10.1039/D4SM00304G
MacKay, J., Hart, L. R., Tareq, A. Z., Wang, S., Gonzalez Abrego, V., Maskery, I., Irvine, D., Wildman, R. D., & Hayes, W. (2025). 4D printed polymethacrylate lattices capable of dimensional switching and payload release via photoresponsive actuation of azobenzene units. Materials Advances. https://doi.org/10.1039/D5MA00670H
Cheng, Y. et al. (2025). Development of novel multi-responsive 4D printed smart nanocomposites with polypyrrole coated iron oxides for remote and adaptive transformation. Materials Horizons. https://doi.org/10.1039/D4MH01804D
