Petrol vs Ethanol Fuels: Impact on Engine Performance and Materials Compatibility
By Mufaddal Shakir
8/31/20254 min read
As India and many other countries are moving towards ethanol blending in petrol (E10, E20, and beyond), it’s important for vehicle owners to understand how ethanol behaves differently from petrol. While ethanol is being promoted as a cleaner and renewable alternative, it also brings certain challenges in terms of vehicle performance and materials compatibility.
In this article, we’ll explore the differences between petrol and ethanol fuels, their impact on engine performance, and the material upgrades required to make vehicles ethanol-compatible.
1. Petrol vs Ethanol: A Deeper Look at Fuel Properties
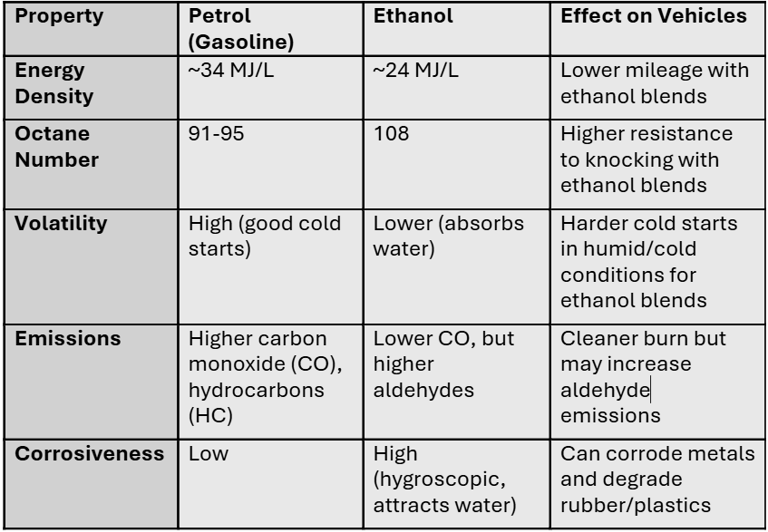
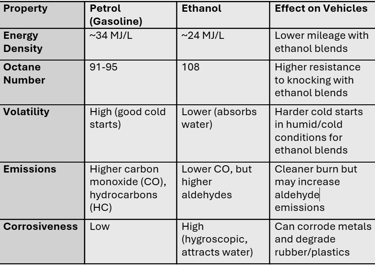
Petrol (gasoline) is a hydrocarbon mixture derived from crude oil. Its molecular structure is non-polar, which means:
It doesn’t easily absorb water.
It has high energy density (~34 MJ/L), leading to better mileage.
It has a moderate octane rating (91–95) which makes it susceptible to knocking at high compression ratios.
Ethanol (C₂H₅OH), on the other hand, is an alcohol with a hydroxyl group (-OH).
It is hygroscopic in nature, meaning it absorbs moisture from air.
It has low energy density (~24 MJ/L), meaning more volume of fuel is needed to travel the same distance.
It has a very high octane rating (~108), which is excellent for preventing knocking in high-compression or turbocharged engines.
We can say that:
Petrol provides better mileage.
Ethanol provides better anti-knock performance, enabling engines to run at higher compression ratios (which could theoretically recover some efficiency losses if engines were specially tuned for ethanol).
2) Effect on Engine & Vehicle Performance
a) Mileage and Power
Ethanol has 30% less energy per litre compared to petrol.
A 20% blend (E20) typically reduces mileage by ~5–7%.
Engine power is only slightly affected (1–2% drop), often compensated by higher octane rating.
b) Combustion & Emissions
Ethanol fuel burns cleaner, meaning less CO emissions.
May produce more aldehydes (formaldehyde, acetaldehyde).
Engines may run slightly hotter due to faster flame speed.
c) Starting & Driveability
Cold starts can be harder (especially below 15°C).
Ethanol absorbs moisture which increases the risk of phase separation in fuel tanks.
3) Starting & Driveability
Cold starting issues with ethanol come from its low volatility and water-absorbing tendency:
Petrol vaporizes easily at low temperatures, helping the air–fuel mixture ignite quickly.
Ethanol requires higher heat for vaporization, which means in colder climates (or humid monsoons in India), the fuel-air mix may not ignite efficiently.
Another issue is phase separation:
If ethanol absorbs too much water, the mixture separates into two layers, water + ethanol and petrol.
When this separated fuel enters the engine, it causes misfires, poor acceleration, and corrosion inside the fuel system.
So while driveability with E20 is acceptable in tropical countries like India, colder regions may experience rough starts, hesitation during acceleration, and inconsistent idling if engines are not tuned properly.
4) Why Materials Matter in Ethanol Compatibility?
The chemical reactivity of ethanol compared to petrol is the core reason for material degradation:
Metals: Ethanol + water leads to the formation of weak acidic solutions that corrode aluminum, zinc, and mild steel.
Rubbers/Plastics: Ethanol molecules are polar and small, thus they penetrate polymer chains, causing swelling, softening, or cracking.
This is why fuel system materials must be upgraded:
Elastomers like NBR (nitrile) and neoprene absorb ethanol, swell, and lose elasticity, leading to leaks.
Metals like aluminum carburetors corrode, affecting fuel metering.
Fuel lines made of natural rubber can harden and crack.
Thus, materials science is central to ethanol adoption. Without proper materials, engines would suffer frequent breakdowns even if the combustion side works fine.
5) Materials Upgrade: Making Vehicles E20-Compatible
a) Fuel Tank:
Mild steel rusts quickly when ethanol attracts water.
Stainless steel (304/316) contains chromium, forming a passive oxide layer that resists corrosion.
HDPE multilayer tanks use EVOH (ethylene-vinyl alcohol) as a barrier layer, preventing ethanol from permeating through plastic walls and avoids vapor loss.
b) Fuel Lines & Hoses:
Viton (FKM) has high fluorine content which provides excellent chemical resistance.
PTFE (Teflon) is inert, has low permeability which means that ethanol cannot diffuse through. This prevents swelling, cracking, and leaks.
c) Seals & Gaskets:
Ethanol degrades NBR by softening and reducing tensile strength.
HNBR (Hydrogenated NBR) resists polar solvents better.
Silicone (VMQ) remains flexible across temperatures, maintaining sealing ability.
d) Fuel Pump and Injectors:
Small moving parts are most vulnerable because ethanol carries tiny water droplets.
Stainless steel and nickel-plated alloys resist pitting corrosion.
Engineering polymers (PEEK, PPS) are used for impellers and housings because they don’t degrade in ethanol-rich environments.
e) Carburetor/Throttle Body:
Old zinc alloys react with ethanol-water mixtures, leading to powdery corrosion that clogs jets.
Hard anodized aluminum builds a dense oxide layer, resisting fuel corrosion.
Ethanol-resistant polymer floats prevent swelling and sinking.
In summary, the shift from standard rubbers and cheap alloys to fluoropolymers, engineering plastics, and stainless steels is what makes vehicles ethanol-ready.
6) Practical Implications for Riders & Drivers
Fuel Efficiency: Expect a slight drop in mileage of approximately 5–7%.
Maintenance: Spark plugs, injectors, and fuel filters may require more frequent inspection and service.
Long-Term Use: Vehicles which are not designed for E20 blends may suffer from hose cracking, seal failures, or injector corrosion.
Storage: Ethanol-blended fuels degrade faster. One should avoid storing fuel for long periods.
7) The Road Ahead
India plans to roll out E20 fuel nationwide by 2025–26. Vehicle owners should check if their model is E20-ready (often indicated by a sticker near the fuel cap). For older vehicles, material retrofits may be necessary to avoid long-term damage.
While ethanol-blended fuel supports energy security, lower emissions, and reduced oil imports, its successful adoption depends heavily on materials engineering, ensuring that the metals, rubbers, and plastics inside our vehicles can withstand the chemistry of ethanol.
Petrol and ethanol blends represent the future of greener mobility, but their impact on performance and durability boils down to the science of materials. As automotive engineers and enthusiasts, understanding this helps us prepare for a smooth transition into the E20 era.
